Enzyme Energy Analysis Needed
Note to Self and Joe D.
This post was written as a “note-to-self” lamenting whether or not the enzyme Aconitase is a machine in the sense described in the referenced paper I recently wrote. The claim made in the paper is that some enzymes are machines and function differently than normal chemical reactions, including those involving catalysts – that enzyme action is a different paradigm. The paper discusses what machines are, from an engineering point of view, and watching the video showing the Aconitase enzyme in action shows some, but not all what I expected based on the characteristics of what I define as a “machine”.
Do Molecular Machines Always Require Added Energy?
In the paper “On the Limits of Natural Causes” the claim is made that all machines require logical functionality enabled by energy. But is energy always lost, that is, not recovered? The reason this question arises is video 2 in the paper which is an animation of the Aconitase enzyme converting a citrate molecule to an isocitrate molecule. These two molecules have the same atoms and types of bonds, so it would seem that the bond energy and entropy of the molecules is the same. There is no hint that any expended ATP molecules for the enzyme to facilitate the conversion which suggests that the claim is not always true in the lossless molecular world. The claim is based on the concept that machines perform specified work which requires logical functionality which requires energy expenditure. An energy analysis of this enzyme’s operation should answer this question. However, knowing that the entropy and enthalpy are the same both before and after the conversion is possibly proof that no energy was lost during the process.1
A colleague suggested that the starting and ending entropy are different that accounts for the energy lost due to logical functionality. But I am not convinced.2 The Aconitase enzyme is required to create isocitrate molecules when needed, and building the enzyme does take materials and energy. So the question (do some enzymes not consume energy to power their logical functionality?) posed here does not impact the arguments made in the paper, that is, that intelligence requires expenditure of energy that otherwise (in the natural world, no intelligence involved} would not be necessary. The paper would require some adjustment to the intelligent work definition that makes an exception for some molecular enzymes whose intelligence is embedded in the specified mechanical configuration of the enzyme. And the explanation that sometines the logical energy is recoverable without loss would need to be added. However, the fact remains that the specified configuration of the enzyme molecule is a requirement to achieve a timely conversion of the citrate molecule to an isocitrate molecule.
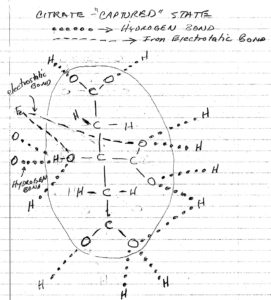
Figure 1. Citrate, w/electrostatic bond detail
Figure 1 is the chemical equation for the citrate molecule with the electrostatic bonds shown in the video added, shown as dash and dotted lines. My original thought was that if the magnitude of these bond energies could be calculated for each state of the process, one could determine if energy was conserved or not. But on second thought, it would seem that one is making and breaking the same bonds, therefore the net sum would be zero.3
Assuming the transformation of the citrate to isocitrate molecule is achieved without any free energy change to the system, the next question is: what causes the each of the next process steps to take place? The logical answer (to me) is that each step establishes new initial conditions that provide a singular, specified state change with the available free energy. If this is true, then the enzyme design is very, very sophisticated! The last state change would have to “eject” the newly formed isocitrate molecule.
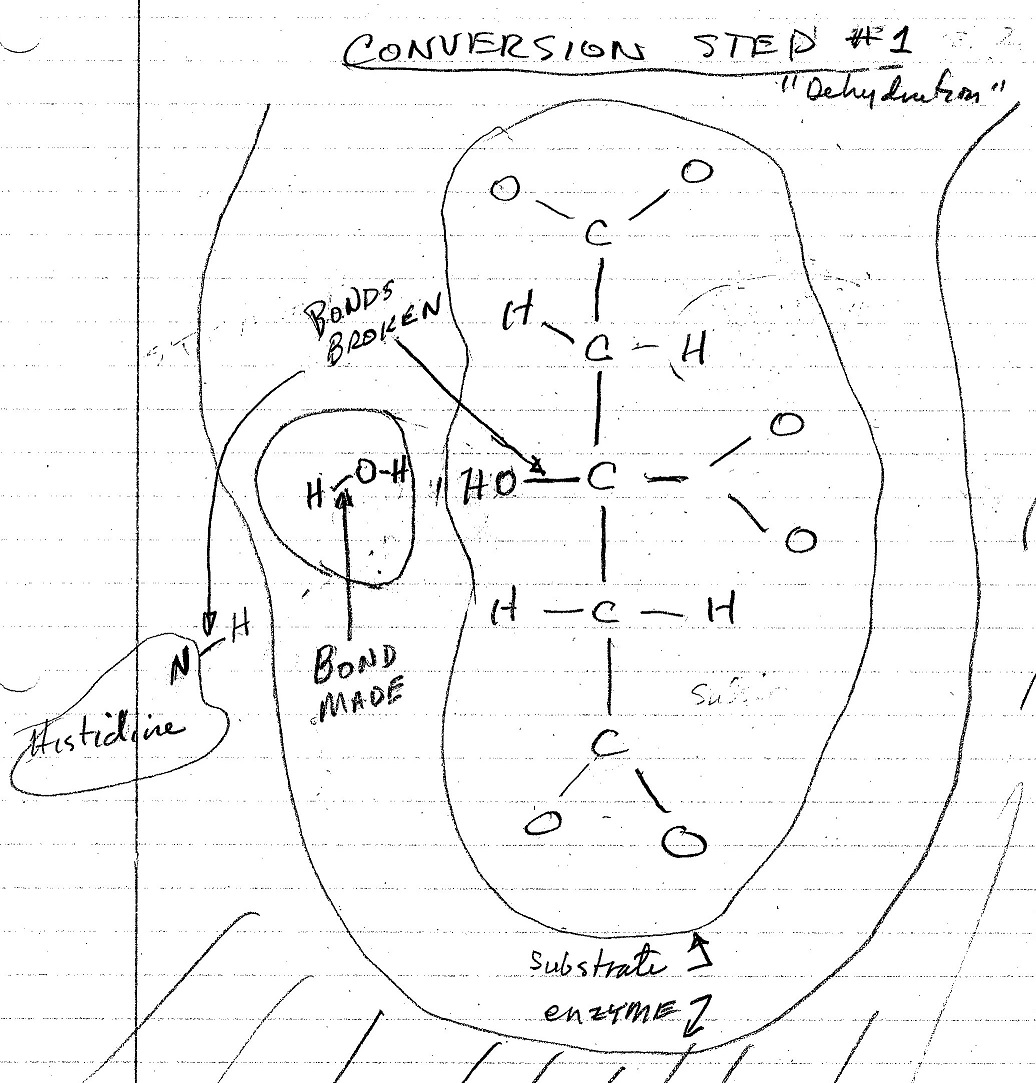
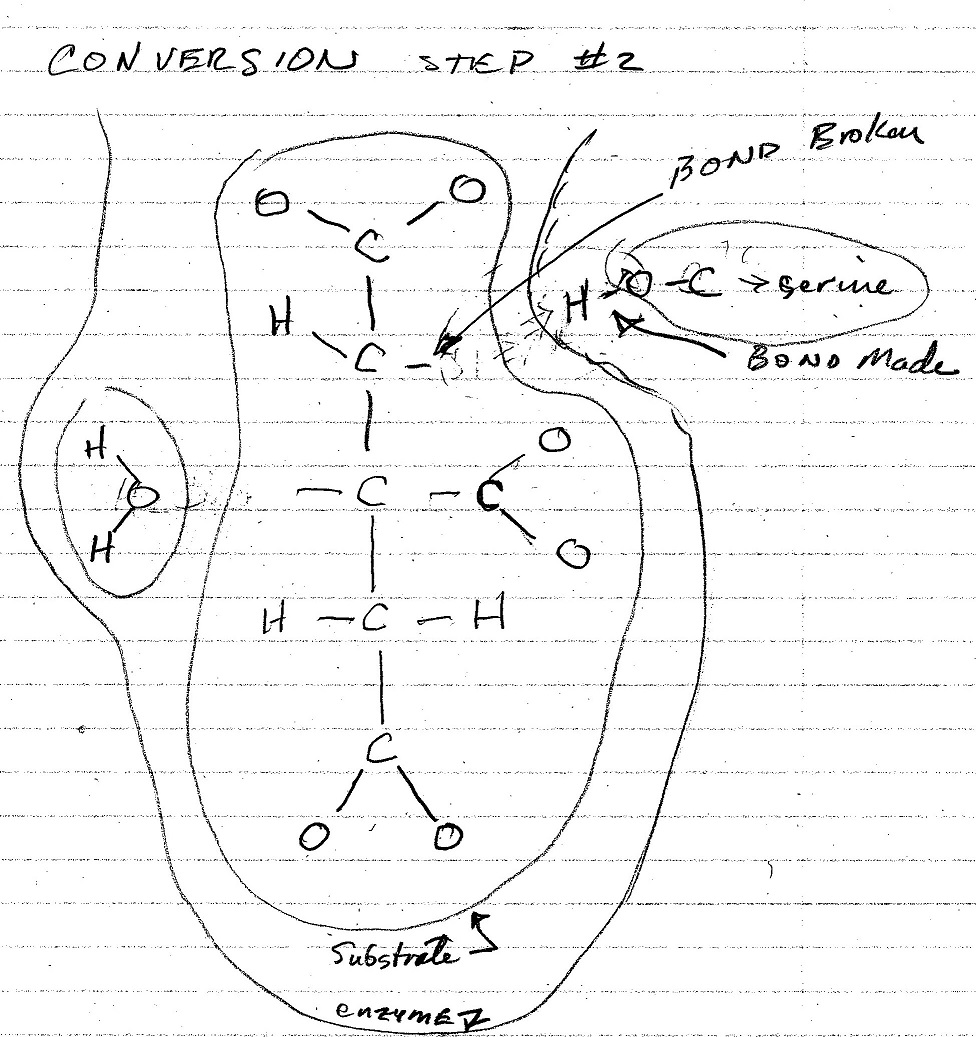
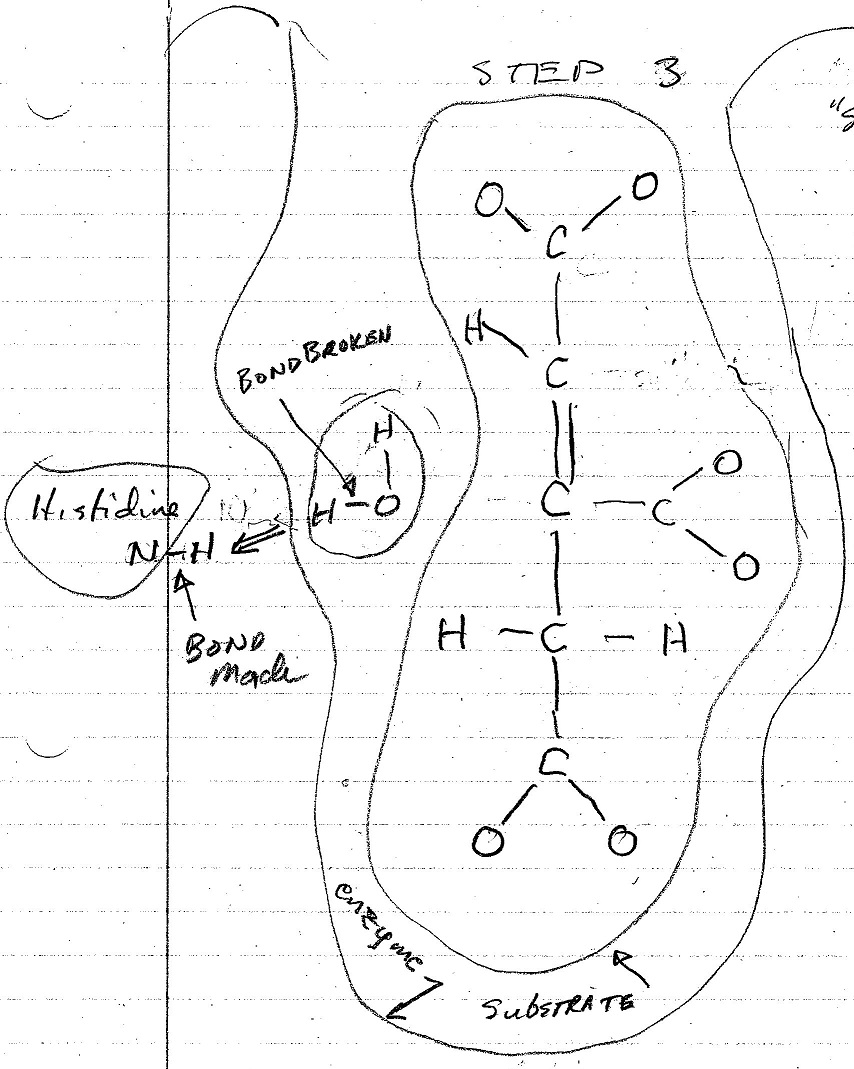
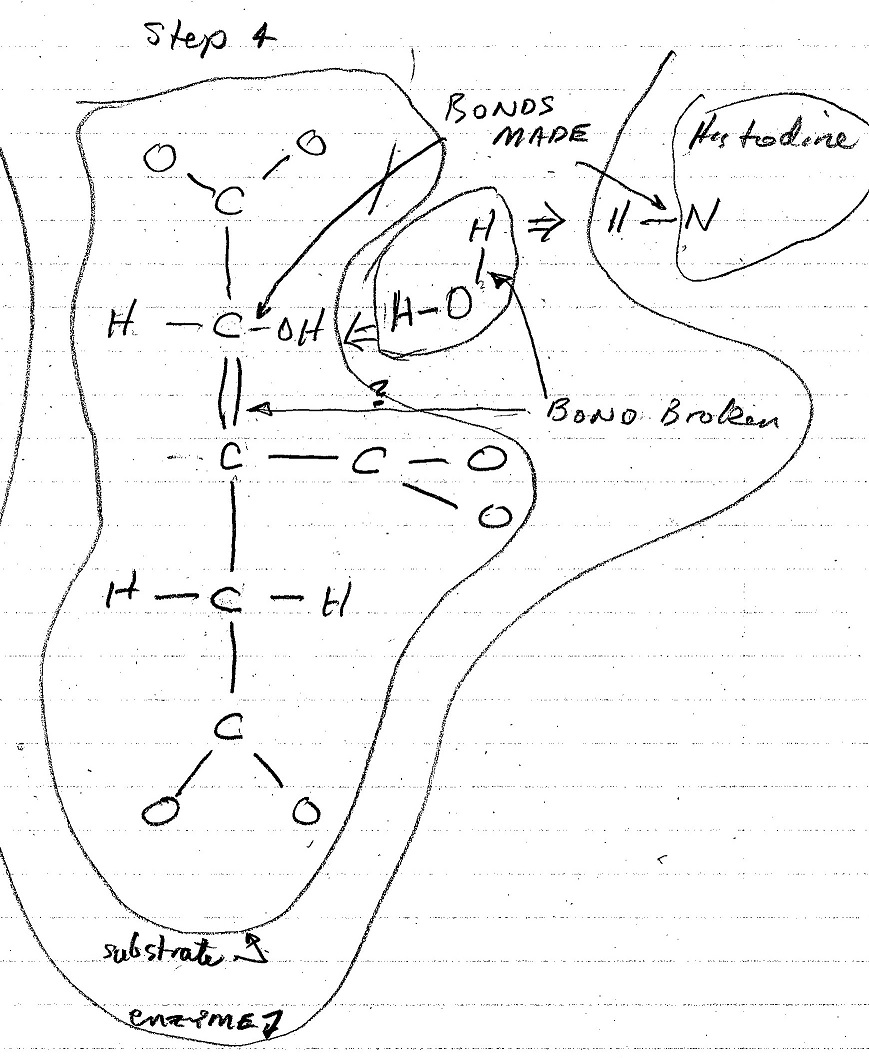
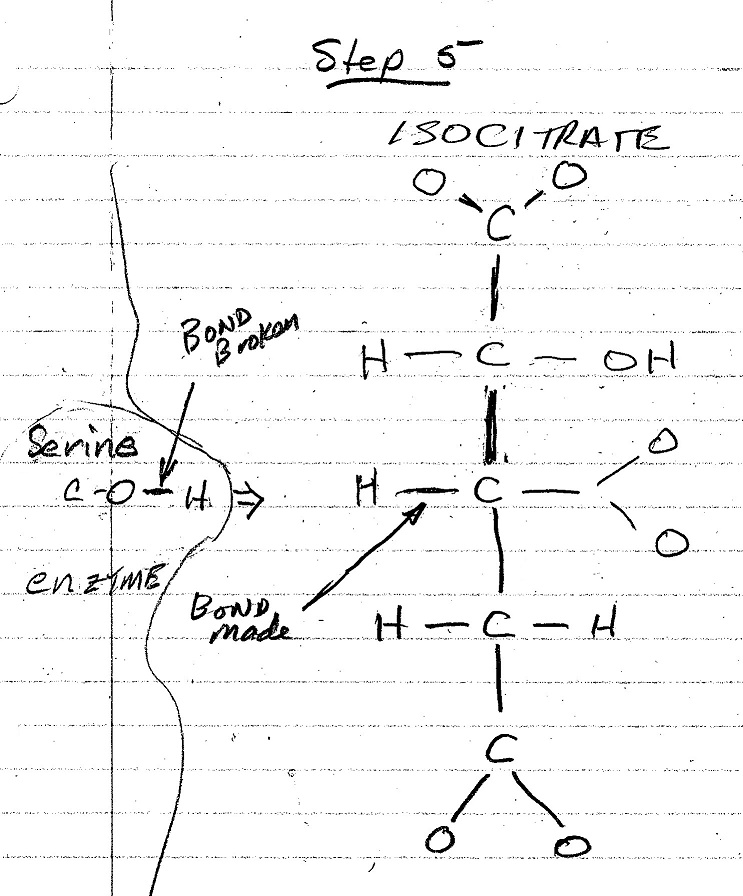
The figures below are my interpretation of the covalent bonds made and broken at each step of the conversion process as shown in the video. The emzine amino acid involved in these interactions is identified, and the water molecule that is formed in the process is also shown. The electrostatic forces involved in each of these stages is not shown in the video nor shown in these figures.